Recently, the research group led by Professor Lu Ming from the Department of Pharmacology, School of Basic Medical Sciences, published a research article in Acta Pharmaceutica Sinica B titled “Astrocyte FGF7/FGFR2 Autocrine Signaling Mediates Neuroinflammation and Promotes MPTP-Induced Degeneration of Dopaminergic Neurons.” For the first time, the study,identified FGF7 as an “accelerator” of astrocyte inflammatory responses and β-Arrestin2 as a “brake” suppressing neuroinflammation. The imbalance between FGF7/FGFR2 autocrine signaling and DRD2/β-Arrestin2-biased signaling amplifies inflammatory effects and drives a positive feedback loop of dopaminergic neuron degeneration. The team successfully utilized a novel compound, the β-Arrestin2-biased agonist, UNC9995, to restore the “brake” function and block the uncontrolled “accelerator,” opening a new avenue for neuroprotective therapy in Parkinson’s disease (PD).

Reactive astrocytes exhibit both neuroprotective and neurotoxic phenotypes and are involved in the onset and progression of various central nervous system diseases. Promoting the transition of astrocytes toward a neuroprotective phenotype, or inhibiting their transition to a neurotoxic phenotype, represents a promising strategy for developing disease-modifying treatments for neurological disorders. In PD, a large number of neurotoxic astrocytes mediates and amplifies neuroinflammatory responses, thereby accelerating dopaminergic neuron degeneration. The heterogeneity of inflammatory astrocytes is significant, encompassing multiple subtypes, yet the key molecules regulating their phenotype transition have remained unknown. Through transcriptomic sequencing of primary astrocytes from β-Arrestin2 knockout mice, the researchers found significantly elevated expression of fibroblast growth factor FGF7, a member of the fibroblast growth factor family. Further studies using β-Arrestin2 knockout mice and various astrocyte-specific conditional knockout mice confirmed that the imbalance between DRD2/β-Arrestin2-biased signaling and FGF7/FGFR2 autocrine signaling is a critical factor in driving inflammatory phenotype transition in astrocytes and dopaminergic neuron degeneration in PD. Additionally, the team used a novel β-Arrestin2-biased agonist, UNC9995, to successfully activate DRD2/β-Arrestin2-biased signaling, thereby suppressing astrocyte inflammatory responses and preventing dopaminergic neuron degeneration, providing a promising new strategy for anti-inflammatory therapy in PD.
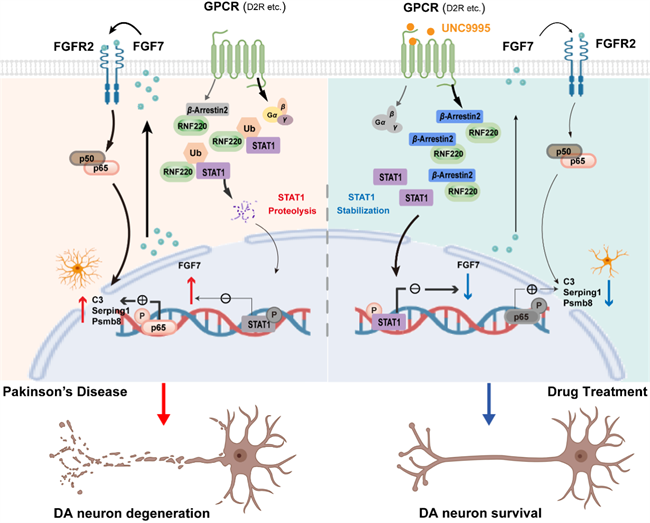
This study elucidates the molecular mechanism by which FGF7/FGFR2 autocrine signaling and DRD2/β-Arrestin2-biased signaling bidirectionally regulate the inflammatory phenotype transition in astrocytes, offering a novel target combination for anti-inflammatory therapy in PD.
Professor Lu Ming, Professor Hu Gang, and Associate Professor Cao Lei from the Department of Pharmacology, School of Basic Medical Sciences, are co-corresponding authors of the paper. The research was supported by the Ministry of Science and Technology’s 2030 “Brain Science and Brain-Inspired Research” Major Project, the National Natural Science Foundation of China, and other funding sources.
Original article link: https://doi.org/10.1016/j.apsb.2025.07.012
(Drafted by Lu Ming’s Research Group; Reviewed by Chen Feng and Wang Juejin; Translation revised by Zhang Bei)



