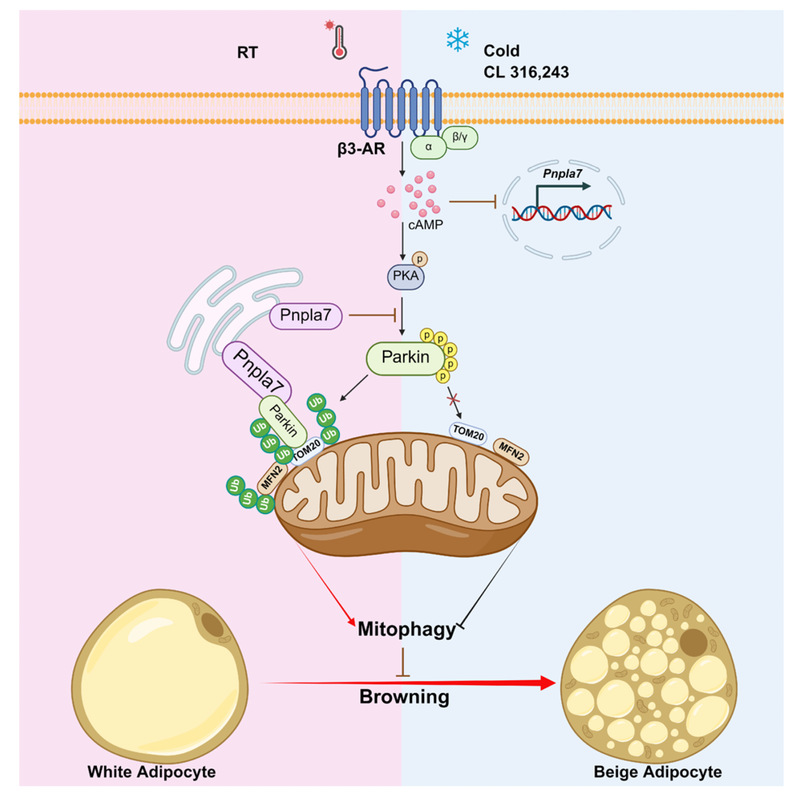
Recently, the research team led by Professor John Zhong Li, Director of the Jiangsu Provincial Key Laboratory of Molecular Targets and Interventions for Metabolic Diseases at the School of Basic Medical Sciences, Nanjing Medical University (NMU), published a research paper in Nature Communications titled “PNPLA7 mediates Parkin-mitochondrial recruitment in adipose tissue for mitophagy and inhibits browning ”. This study reveals that PNPLA7 in white adipose tissue inhibits PKA-mediated phosphorylation of Parkin through interaction with Parkin, enhances Parkin translocation to mitochondria, and promotes mitophagy, thereby suppressing the browning of white adipose tissue. In contrast, knockout of PNPLA7 in white adipose tissue reduced Parkin mitochondrial translocation, suppressed mitophagy, and promoted adipose tissue browning. These findings elucidate a previously unrecognized mechanism underlying the regulation of mitophagy during cold-stimulated white adipose browning.

During cold stimulation, mitophagy plays a pivotal role in maintaining mitochondrial homeostasis in both brown and beige adipocytes. The PINK1/Parkin-mediated pathway is a widely characterized, ubiquitin-dependent mechainsm of mitophagy . Studies have shown that Parkin-mediated mitophagy is downregulated in iWAT browning and is essential for the transition of beige adipocytes to white adipocytes (a process known as whitening) upon the withdrawal of external stimuli. Parkin deletion specifically in adipose tissue protects mice against high-fat diet and aging-induced obesity by coordinating mitophagy with mitochondrial biogenesis in white adipocytes.
Furthermore, norepinephrine-induced activation of β3 adrenergic receptor (β3-AR) has been shown to inhibit Parkin mitochondrial recruitment through PKA-mediated phosphorylation, thereby reducing mitophagy and promoting browning of WAT. However, the molecular events by which Parkin is recruited to mitochondria in WAT has not been fully elucidated. This study, employing multiple mouse models, reveals for the first time that PNPLA7--an ER and mitochondrial-associated membrane (MAM) protein--as a critical regulator in maintaining energy homeostasis and browning processes within white adipose tissue by modulating mitophagy. These findings identify that PNPLA7-mediated Parkin’s mitochondrial recruitment and mitophagy is a critical regulatory module to control iWAT browning and suggest a promising pharmaceutical target for the treatment of obesity and related metabolic disorders.
Professors John zhong Li and Qian Wang from the Jiangsu Provincial Key Laboratory of Molecular Targets and Interventions for Metabolic Diseases at the School of Basic Medical Sciences, NMU, are the co-corresponding authors of the paper. Dr. Xuetao Ji and Xu Zhang, also from the School of Basic Medical Sciences, are the co-first authors. This research was supported by the National Natural Science Foundation of China, the National Key Research and Development Program of the Ministry of Science and Technolgoy of the PRC, and the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine of Jiangsu Province.
The research group is actively recruiting postdoctoral researchers. Interested candidates are warmly welcome to join the lab.
Lab recruitment information:
https://jcyxy.njmu.edu.cn/2014/0317/c2185a29819/page.htm
Link to the original paper:
https://www.nature.com/articles/s41467-025-61904-w
(Drafted by John Zhong Li’s research team; Translation revised by Zhang Bei)



