Recently, the research team led by Professor Xiuxing Wang from the School of Basic Medical Sciences at Nanjing Medical University (NMU), in collaboration with Professor Wei Zhang from Beijing Tiantan Hospital, Capital Medical University, Professor Jeremy N. Rich from the University of Pittsburgh Medical Center, and Professor Yongping You from the Department of Neurosurgery at the First Affiliated Hospital of NMU, published a groundbreaking study in NatureCommunications titled “Combined Targeting of Glioblastoma Stem Cells of Different Cellular States Disrupts Malignant Progression.” The study reveals that glioblastoma patients with a higher proportion of classical (CL) and mesenchymal (MES) glioblastoma stem cells exhibit poorer survival outcomes. The findings suggest that a therapeutic stratey combining targeted inhibition of both CL and MES glioblastoma stem cells may serve as a promising novel approach for treating glioblastoma.

Glioblastoma (GBM) is an aggressive and highly lethal primary malignant tumor of the central nervous system. Glioblastoma stem cell (GSC), a subpopulation of cells within GBM with self-renewal and multi-lineage differentiation capacities, are considered the “seeds” of tumor initiation, recurrence, and treatment resistance. Previous studies have often treated GSC as a homogeneous population, identifying them based on specific markers. However, estalished GSC markers exhibit heterogeneous transcriptional profiles, suggesting intrinsic GSC heterogeneity. The biological characteristics and functional mechanisms underlying GSC heterogeneity remain largely unexplored.
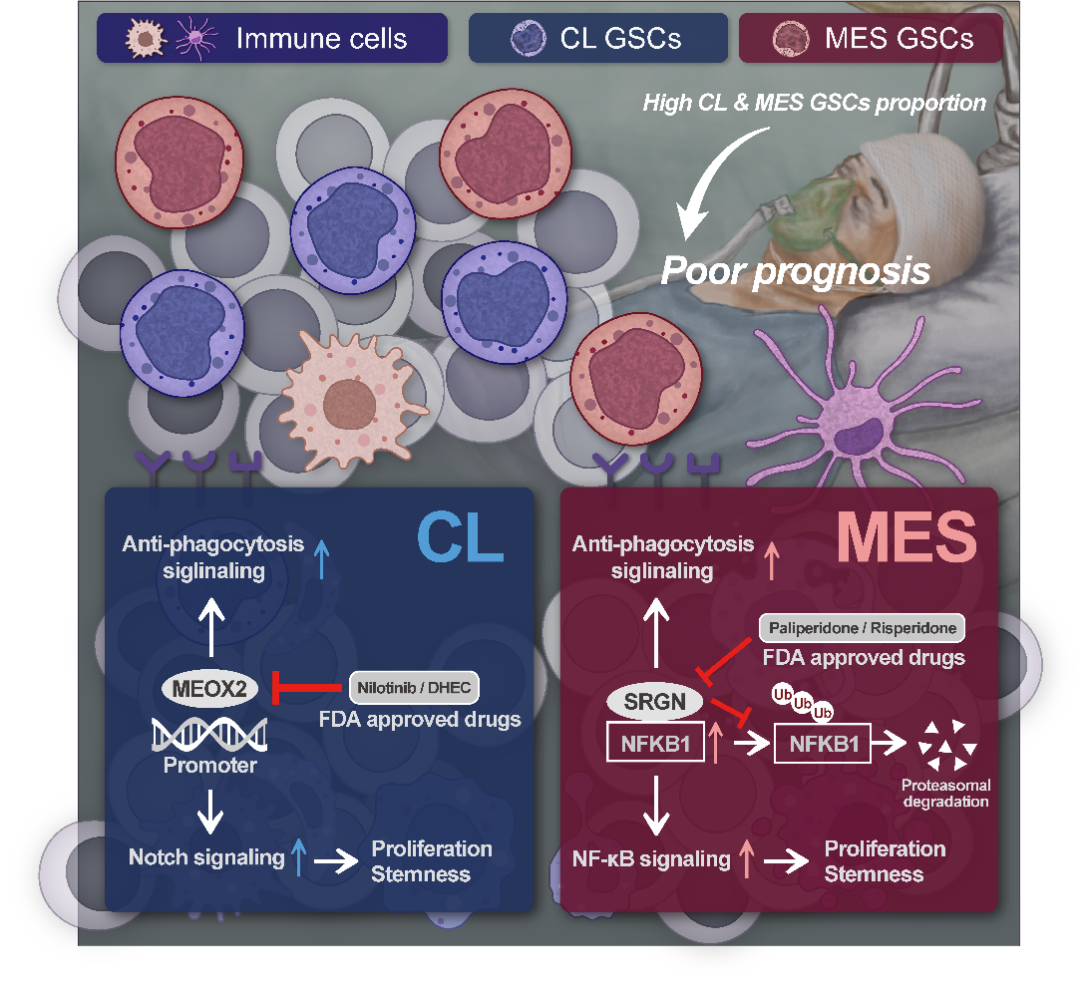
Through integrated multi-omics analysis of single-cell transcriptomes and GSC transcriptomic profiles from GBM patients, the research team discovered that different patients exhibit varying proportions of molecular GSC subtypes. Notably, . patients with a higher proportion of classical (CL) and mesenchymal (MES) GSC subtypes had worse survival outcomes. Further multi-omics analyses identified MEOX2 as a key molecular target for CL GSC and SRGN as a key molecular target for MES GSC. In CL GSC, MEOX2 maintains stemness and the CL subtype through regulation of the Notch signaling pathway. In MES GSC, SRGN stabilizes NFKB1 protein and regulates the NF-κB signaling pathway, thereby sustaining stemness and the MES subtype. Through virtual molecular screening and validation via in vitro and in vivo experiments, the study identified two FDA-approved drugs--nilotinib and paliperidone--as potential theraputic agents targeting MEOX2 and SRGN, respectively. The efficacy of this combined targeting strategy was further confirmed using a heterogeneous mouse model, which demonstrated that simultaneous inhibition of CL and MES GSCs effectively suppresses malignant tumor progression.
This study provides critical insights into the molecular mechanisms by which MEOX2 and SRGN contribute to maintaining the characteristics of CL and MES GSC subtypes, respectively. Moreover, it introduces a novel therapeutic strategy targeting the heterogeneity of GSC populations in GBM. Based on these findings, an investigator-initiated clinical trial is currently underway to evaluate the clinical feasibility of this approach.
Dr. Chenfei Lu, a postdoctoral fellow from the Department of Neurosurgery at the First Affiliated Hospital of NMU; Tao Kang,a master's student at the School of Basic Medical Sciences, NMU; Professor Junxia Zhang from the Department of Neurosurgery at the First Affiliated Hospital of NMU; Dr. Kailin Yang from the Cleveland Clinic; and Associate Professor Yang Liu from the School of Medicine at Nanjing University of Chinese Medicine served as co-first authors of the study. The study was jointly led by Professor Xiuxing Wang from the School of Basic Medical Sciences at NMU, Professor Wei Zhang from Beijing Tiantan Hospital of Capital Medical University, Professor Jeremy N. Rich from the University of Pittsburgh Medical Center, and Professor Yongping You from the Department of Neurosurgery at the First Affiliated Hospital of NMU. This research was supported by the National Young Talents Program, the National Natural Science Foundation of China, the Jiangsu Provincial Natural Science Foundation, and the Jiangsu Distinguished Professorship Program.
Article Link:
https://www.nature.com/articles/s41467-025-58366-5
(Drafted by Wang Xiuxing’s Research Team; Reviewed by Chen Feng and Wang Juejin; Translation reviesed by Zhang Bei)



