The phenomenon that macrophages polarize to different phenotypes with different function is a major driver of atherogenesis, which usually includes the onset of polarization at the M0 phase (resting state), the M1 phase (pro-inflammatory, classically activated macrophages), and the M2 phase (anti-inflammatory, alternatively activated macrophages). However, whether macrophage polarization is characterized by epigenetic histone modification dynamics has not been clearly reported. Here, Professor Hongshan Chen’s group found a new epigenetic mechanism about the macrophage polarization in atherosclerosis

In the present study, the findings from bioinformatic analysis and multiple experimental explorations demonstrated that histone modifications play different regulatory roles in the pro- and anti-inflammatory processes of macrophages. M0 macrophage is dominated by the maintenance of histone methylation modification system. Histone methylation and acetylation modifiers change significantly during the transition of macrophages from M0 to M1, while it was mainly histone lactylation-related modifiers that dominated when macrophages shifted to M2 macrophages. Particularly, the most dramatic change of histone lactylation is marked by H3 lysine 18 lactylation.
Further, we found that this process is closely related to the nuclear chromatin remodeling triggered by the macrophage energy reprogramming product lactate, becoming an important indicator of cellular homeostatic shifts and repair. Stress induced energy reprogramming and changed MCT4 expression (an important channel for lactate efflux from the cell), leading to lactate fluctuation. MCT4 deficiency contributed to the intracellular lactate accumulation. This sensed P300 to catalyze the formation of H3K18la, which remodeled the nuclear chromatin microenvironment and specifically activated the transcription of anti-inflammatory genes and the TCA cycle genes. Therefore, this process resulted in the initiation of local repair and homeostasis, and inhibited atherosclerosis. This novel epigenetic mechanism is a new target for atherosclerosis protection and repair, which was also preliminarily developed and validated in this study. Our study provided a basis for future developing novel diagnostic and therapeutic strategies for clinical atherosclerosis.
This work was published in Cell Reports on May 10, 2024. The corresponding authors are professor Hongshan Chen and associate professor Xuesong Li from key laboratory of Cardiovascular and Cerebrovascular Medicine of Nanjing Medical University, and professor Yongfeng Shao from department of Cardiovascular Surgery of the First Affiliated Hospital of Nanjing Medical University. The first authors are Yunjia Zhang, Hong Jiang, Mengdie Dong and Jiao Min. This work is supported by grants from the National Natural Science Foundation of China, Natural Science Foundation of the Jiangsu Higher Education Institution of China and Special Program for Top Innovative Talents et al.
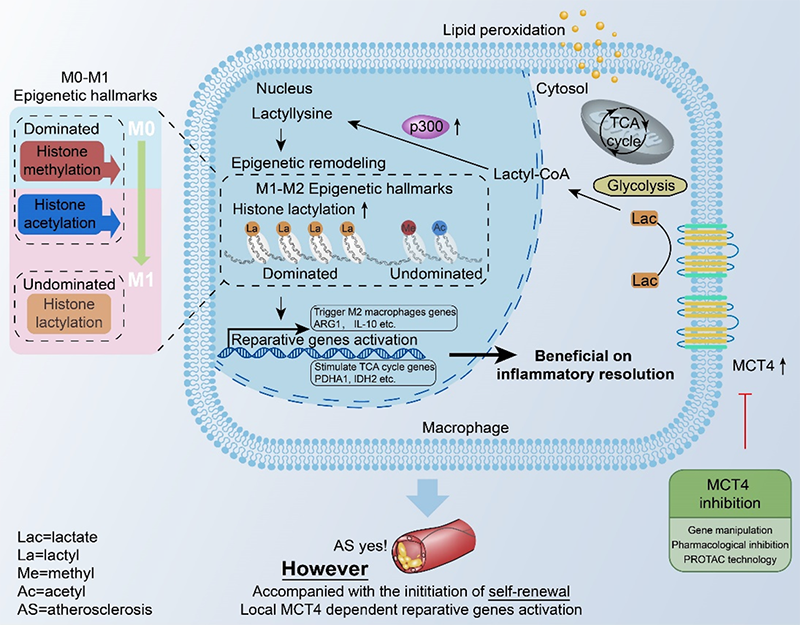
Model:MCT4 deficiency enhances histone H3 lysine 18 lactylation level which favored a reparative environment through improving anti-inflammatory activity and metabolic rewiring, further reducing atherosclerosis progression.
Original publication:https://www.cell.com/cell-reports/pdf/S2211-1247(24)00508-4.pdf



